wilate® is a VWF/FVIII concentrate that is proven to be effective in people with all types of von Willebrand disease for the management of bleeding during surgery, as prophylaxis for the prevention of bleeding, and for on-demand treatment of bleeds1-3
wilate® is a VWF/FVIII concentrate that is proven to be effective in people with all types of von Willebrand disease for the management of bleeding during surgery, as prophylaxis for the prevention of bleeding, and for on-demand treatment of bleeds1-3
wilate® was developed specifically for people with von Willebrand disease (VWD), and contains VWF and FVIII in a physiological 1:1 activity ratio, which may facilitate simple dosing and monitoring even when repeated dosing is needed.4, 5
The purification process includes two virus inactivation steps in order to ensure a high-purity product free of viral contamination.4
Over 15 years of clinical and real-life experience have proven the efficacy and tolerability of wilate® in people with all types of von Willebrand disease for surgery, on-demand treatment and prophylaxis.1-3
*Based on von Willebrand factor and factor VIII activity levels. FVIII, coagulation factor VIII; VWF, von Willebrand factor.
One of the functions of VWF is to bind to and protect FVIII from proteolytic degradation.4
As a result, people with von Willebrand disease may have low levels of both VWF and FVIII, both of which need to be corrected in order to restore normal haemostasis.4
wilate® contains VWF and FVIII in a balanced 1:1 ratio*, which is similar to the activity ratio of VWF and FVIII in the plasma of healthy individuals4
*Based on von Willebrand factor and factor VIII activity levels.
One of the functions of VWF is to bind to and protect FVIII from proteolytic degradation.4
As a result, people with von Willebrand disease may have low levels of both VWF and FVIII, both of which need to be corrected in order to restore normal haemostasis.4
The balanced ratio of VWF and FVIII* may simplify dosing and monitoring of wilate®2
*Based on von Willebrand factor and factor VIII activity levels.
wilate® is a high-purity, human VWF/FVIII concentrate
wilate® is made from pooled human plasma that has been collected in GMP-compliant donation centres, which are regularly inspected by Octapharma and national and international authorities9
FVIII, factor VIII; GMP, good manufacturing practice; VWF, von Willebrand factor.
wilate® is a high-purity, human VWF/FVIII concentrate
All individual plasma samples undergo extensive testing for viruses, including for HIV, hepatitis A, B and C and parvovirus B199, 10
FVIII, factor VIII; HIV, human immunodeficiency virus; VWF, von Willebrand factor.
wilate® is a high-purity, human VWF/FVIII concentrate

wilate® is a high-purity, human VWF/FVIII concentrate
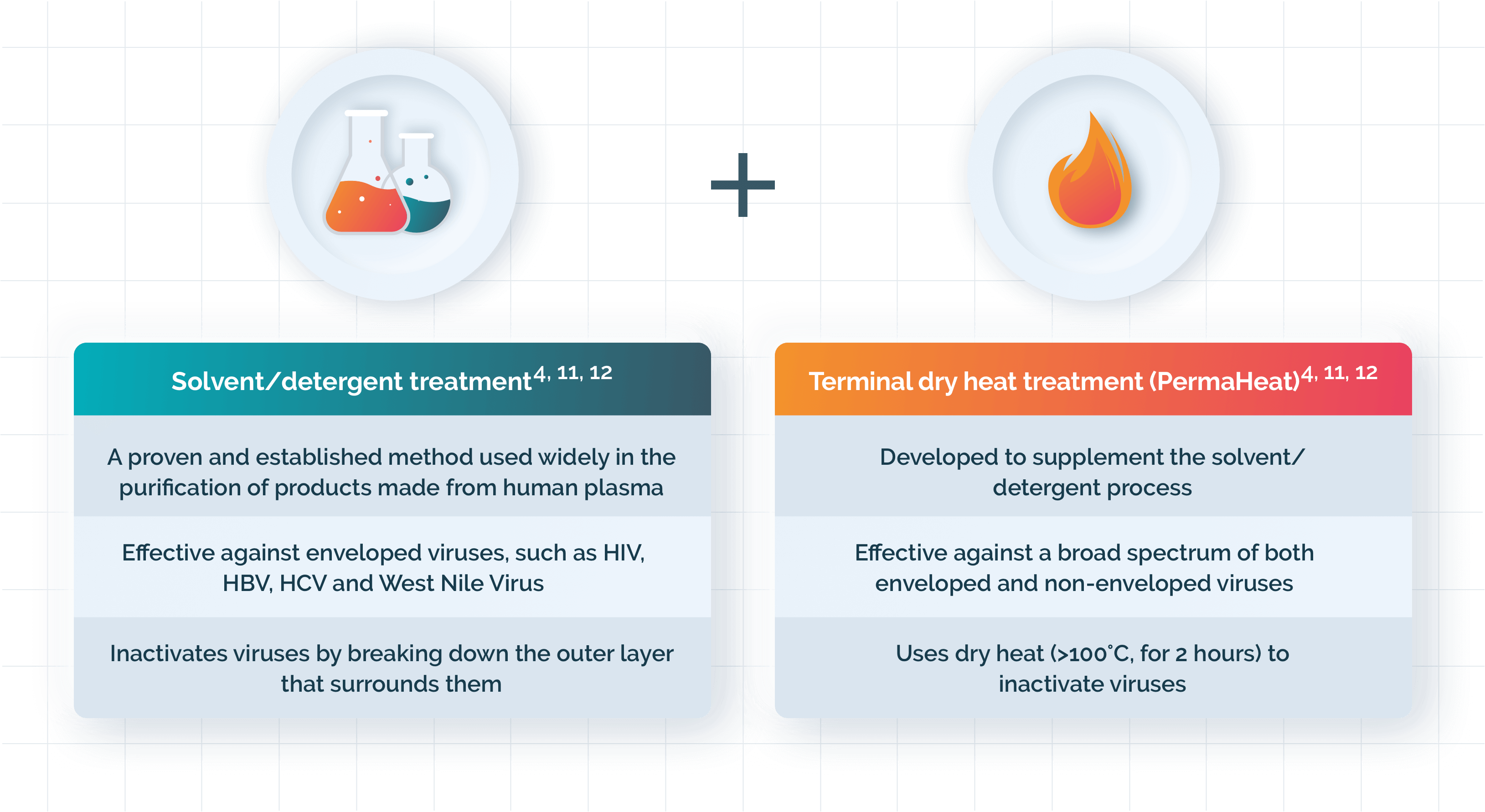
wilate® is purified through a series of carefully controlled processing steps, all designed to minimise impurities and ensure viral safety4
Two chromatographic steps ensure high purity and remove non-essential proteins4
Viral safety is achieved by two complementary virus inactivation steps4
Double virus inactivation process
HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
wilate® is a high-purity, human VWF/FVIII concentrate
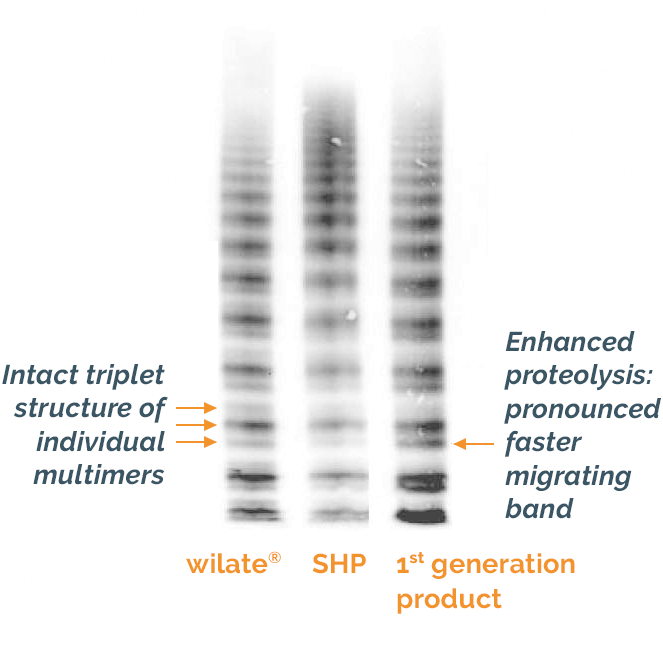
The manufacturing process of wilate® preserves the structure and multimer distribution of VWF seen in the plasma of healthy individuals4
Figure adapted from Windyga et al. 2011 SHP, standard human plasma

The information on this website is not country specific, and may contain information that is outside the approved indications in the country in which you are located. Please contact your local Octapharma representative for the latest information specific to your country.
Please contact your local Octapharma representative for local prescribing information via our contact form.
IMPORTANT
The information on this website is based on the European Summary of Product Characteristics (EU SmPC). US HCPs and other country-specific websites: Below is a list of countries that host a local wilate® website based on local approved information and in local language. Click on the country link to be redirected to the local wilate® website.
The website is provided by Octapharma AG, Seidenstrasse 2, 8853 Lachen, Switzerland
www.octapharma.com
© 2022 Octapharma AG